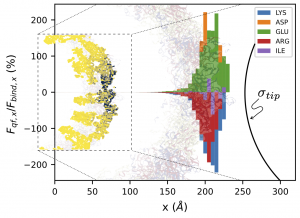
| Electrostatic interactions are crucial for the assembly, disassembly, and stability of proteinaceous viral capsids. Moreover, at the molecular scale, elucidating the organization and structure of the capsid proteins in response to an approaching nanoprobe is a major challenge in biomacromolecular research. Here, the team led by Dr. Guzman reports on a generalized electrostatic model, based on the Poisson-Boltzmann equation that quantifies the subnanometric electrostatic interactions between an AFM tip and a proteinaceous capsid from molecular snapshots. This allows us to describe the contributions of specific amino acids and atoms to the interaction force. We show validation results in terms of total electrostatic forces with previous semi-empirical generalized models at available length scales (d > 1 nm). Then, we studied the interaction of the Zika capsid with conical and spherical AFM tips in a tomography-type analysis to identify the most important residues and atoms, showing the localized nature of the interaction. This method can be employed for the interpretation of force microscopy experiments in fundamental virological characterizations and in diverse nanomedicine applications, where specific regions of the protein cages are aimed to electrostatically interact with molecular sized functionalized inhibitors, or tailoring protein-cage functional properties for nucleic acid delivery. |
 |
Published in: Christopher D Cooper, Ian Addison-Smith, and Horacio V Guzman, “Quantitative electrostatic force tomography for virus capsids in interaction with an approaching nanoscale probe”, Nanoscale, 2022


