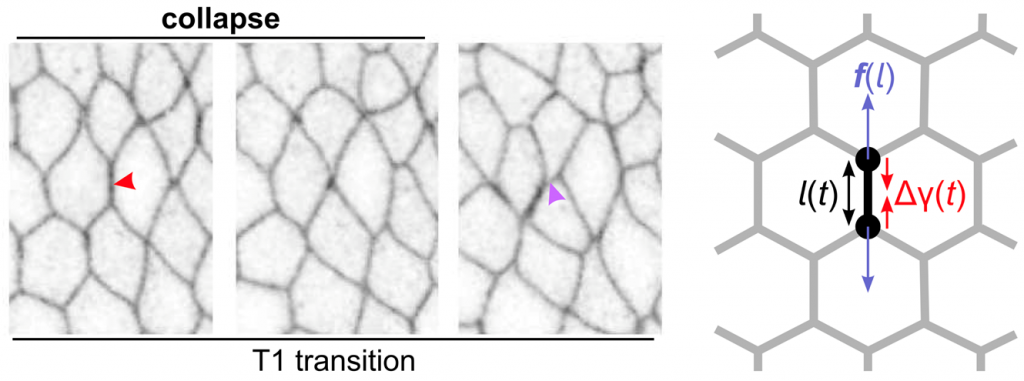
Active cell-junction remodeling is important for tissue morphogenesis, yet its underlying physics is not understood. In their recent paper published in Physical Review Letters, Matej Krajnc and Clément Zankoc studied a mechanical model of cell-cell junctions that describes junctions as dynamic active force dipoles. Their instability can trigger cell intercalations by a critical collapse. In turn, nonlinearities in tissue’s elastic response to local dipoles can stabilize the collapse either by a limit cycle or condensation of junction lengths at cusps of the energy landscape. Furthermore, active junction networks may undergo collective instability to drive active in-plane ordering or develop a limit cycle of collective oscillations, which extends over regions of the energy landscape corresponding to distinct network topologies. These results offer a new view on the dynamics of cell-junction networks during morphogenesis, where large-scale changes of tissue architecture are driven by active processes at a single-cell level.
Publication: M. Krajnc, T. Stern, and C. Zankoc, Phys. Rev. Lett. 127, 198103 (2021).