| The performance of gold nanoparticles (NPs) in applications depends critically on the structure of the NP-solvent interface, at which the electrostatic surface polarization is one of the key characteristics that affect hydration, ionic adsorption, and electrochemical reactions. Using classical molecular dynamics simulations with a polarizable core-shell model for the gold atoms, we demonstrated significant effects of explicit metal polarizability on the solvation and electrostatic properties of gold NPs in aqueous electrolytes. We found considerable spatial heterogeneity of the polarization and electrostatic potentials on the NP surface, mediated by a highly facet-dependent structuring of the interfacial water molecules. The explicit charge response of metal NPs is crucial for the accurate description and interpretation of interfacial electrostatics (e.g., for charge transfer and interfacial polarization in catalysis and electrochemistry). | 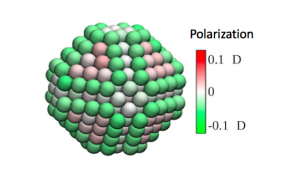 |
Published in:

